What's in your water - and wastewater?
Present in every stream and lake is something invisible, necessary and good. That something is nutrients – chemical compounds that are essential for sustaining aquatic life. However, too much of a good thing can be bad. When nutrient removal isn't considered, treated wastewater discharge into streams and lakes can cause elevated nutrient levels in these surface waters. When nutrient levels remain elevated for an extended period, resulting changes in stream ecology can lead to negative environmental, social and economic impacts.

According to the Merriam-Webster dictionary, a nutrient is “a substance or ingredient that promotes growth, provides energy and maintains life.” In a surface water ecosystem, the primary nutrients are nitrogen and phosphorus. Nitrogen and phosphorus occur in streams and lakes naturally. Nitrogen gas is abundant, making up about 78% of the earth’s atmosphere.
However, most aquatic life cannot use nitrogen gas directly. Instead, they must rely on nitrogen “fixing” organisms to convert nitrogen gas to usable forms of nitrogen. Phosphorus occurs naturally as minerals in rock and sediment and is transported to the water through erosion. Nature does a pretty good job of providing adequate, but not abundant, nutrients for water ecosystems.
Aquatic life such as bacteria, algae and plants use nutrients to grow, providing the base of the food chain for invertebrate and higher life forms. Algae can use sunlight and nutrients to rapidly reproduce.
Excess nutrients in a water ecosystem can cause eutrophication, which is when excessive aquatic plant growth results in the depletion of dissolved oxygen in the water. Low dissolved oxygen can reduce the number and types of beneficial aquatic lifeforms and promote growth of undesirable species. Fish kills, harmful algal blooms and dead zones can all result from low levels to dissolved oxygen because of excess nutrients. The impact of eutrophication is wide-ranging from local to global. Perhaps the local river that once was fun to fish no longer supports game fish. Perhaps recreation in local streams declines because thick algae mats make the water undesirable. At a global level, a hypoxic (low dissolved oxygen) zone or “dead zone” in the Gulf of Mexico covers around 7,000 square miles. The National Oceanic and Atmospheric Administration estimates the economic loss to U.S. seafood and tourism industries caused by the dead zone to be $82 million a year.
Excess nutrients can enter water systems through point sources, such as agricultural runoff from fertilizer application and livestock manure and from point sources such as wastewater treatment and industrial discharges.
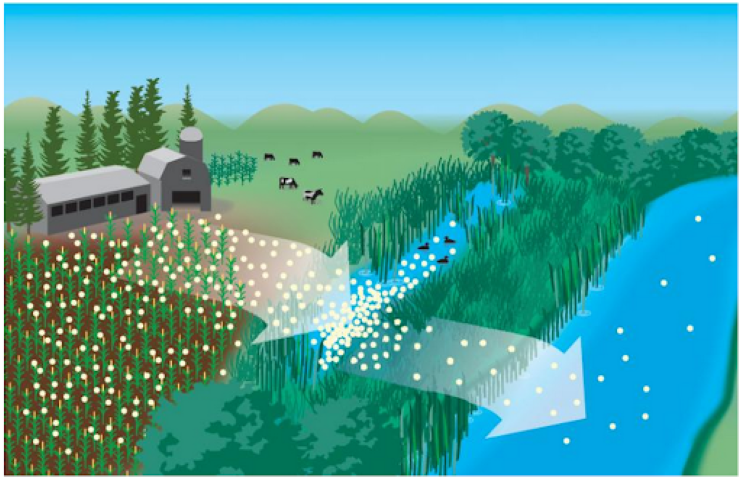
Nitrogen and phosphorus in wastewater originate from human waste, and to a smaller extent food waste and some soaps and detergents. While modern wastewater treatment plants can remove nitrogen and phosphorus from the waste stream, most older treatment plants do not. State and federal requirements have historically not required nutrient removal from wastewater and so earlier designs did not contemplate nutrient removal in the process. Providing the upgrades necessary to remove nutrients can be expensive. However, excess nutrients also carry a high environmental, social and economic cost.
Many states are moving to regulate nutrients in wastewater discharges. Thankfully, wastewater treatment technologies are available to reduce nutrients in the wastewater discharges. Both topics will be discussed in more detail in future posts.
 Lucas Elsbernd, who works in our Manchester, Iowa, office, is a Senior Project
Lucas Elsbernd, who works in our Manchester, Iowa, office, is a Senior Project
Engineer with more than 16 years of experience specializing in water and
wastewater. He’s managed a variety of projects, including site grading, storm
sewer improvements, sanitary sewer lift stations, wastewater collection and
treatment facilities, and water systems and treatment plants. He can be
reached at
Collaborative, Insightful, Results-Driven Solutions
Fehr Graham provides innovative engineering and environmental solutions to help improve the lives and communities of our customers.
